About the INCLUDE project
The INCLUDE Project (Innovations in Clinical Trial Design and Delivery for the Under-served) was one of a suite of large scale projects in key areas of innovation.
The NIHR-INCLUDE project was commissioned in 2017 by the NIHR CRN to address the lack of representation in health and care research - with a vision of providing better health and care through more inclusive research. The NIHR-INCLUDE project finished at the end of 2021 and has now been absorbed as a knowledge resource which can be accessed from the Research Inclusion Toolkits Hub.
About the guidance
This guidance summarises what an under-served group is, a roadmap suggesting intervention points to improve inclusion, examples of under-served groups and barriers to inclusion. It then provides a suggested framework of questions to guide the deliberations of funders, researchers and delivery teams as they design and assess health and care research proposals, and ends with examples of good practice and other resources to guide teams seeking to engage with, and improve inclusion of, under-served groups in health and care research.
The NIHR-INCLUDE Framework and Guidance was the result of a co-design process ensuring they were fit for purpose. Throughout the project we engaged with a diverse range of stakeholders across the research lifecycle which has helped us to reach the audiences NIHR-INCLUDE needed to engage with to initiate the step change in culture required to tackle inclusivity in health and care research.
NIHR-INCLUDE has achieved widespread reach and impact to make health and care research more inclusive. It informs the wider work of the NIHR and is a key cornerstone of the NIHR’s Strategy - ‘Best Research For Best Health: The Next Chapter’.
A detailed description of the work undertaken to inform these guidelines is provided in our publication: Witham M, et al (2020) Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process.
What is an under-served group?
The NIHR-INCLUDE project identified the term ‘under-served’ by diverse stakeholders including patients and the public as the most appropriate term through a consensus workshop. The term has subsequently been adopted by the NIHR and more widely. The term reflects the perspective that the research community needs to provide a better service for people in these groups – the lack of inclusion is not due to any fault of the members of these groups. The term ‘under-served’ reminds us of this perspective in a way that alternative terms such as ‘underrepresented’ do not.
The work of the NIHR-INCLUDE project shows that there is no single definition for an under-served group. Some key characteristics that are common to several under-served groups are:
- Lower inclusion in research than one would expect from population estimates
- High healthcare burden that is not matched by the volume of research designed for the group
- Important differences in how a group responds to or engages with healthcare interventions compared to other groups, with research neglecting to address these factors
The key idea here is that the definition of ‘under-served’ is highly context-specific; it will depend on the population, the condition under study, the question being asked by research teams, and the intervention being tested. No single, simple definition can encompass all under-served groups.
Why is it important to include under-served groups in clinical research?
There are several reasons why it is important to ensure that under-served groups are included in health and care research.
- Failing to include a broad range of participants means that results may not be generalisable to a broad population
- Different groups may respond differently to an intervention due to differences in physiology or disease state. Only by studying the effects of an intervention in a range of groups can we be sure that the balance of risk and benefit is favourable for a given group
- If clinicians lack evidence of the effect of an intervention for a particular group, they may be reluctant to offer the intervention to that group – clinical opinion then takes the place of evidence
- Successful delivery of intervention to target populations is complex, with logistical, sociocultural, psychological and biological differences all having an impact. Unless we have tested if an intervention can be deployed effectively to different groups, we cannot be sure that it will work in practice.
Finally, the principle of ‘no decision about me, without me’ provides the moral justification for ensuring that under-served groups are included in research. The evidence base necessary for decision making by clinicians and patients must be one generated by the participation of a broad range of groups in the research underpinning that evidence base.
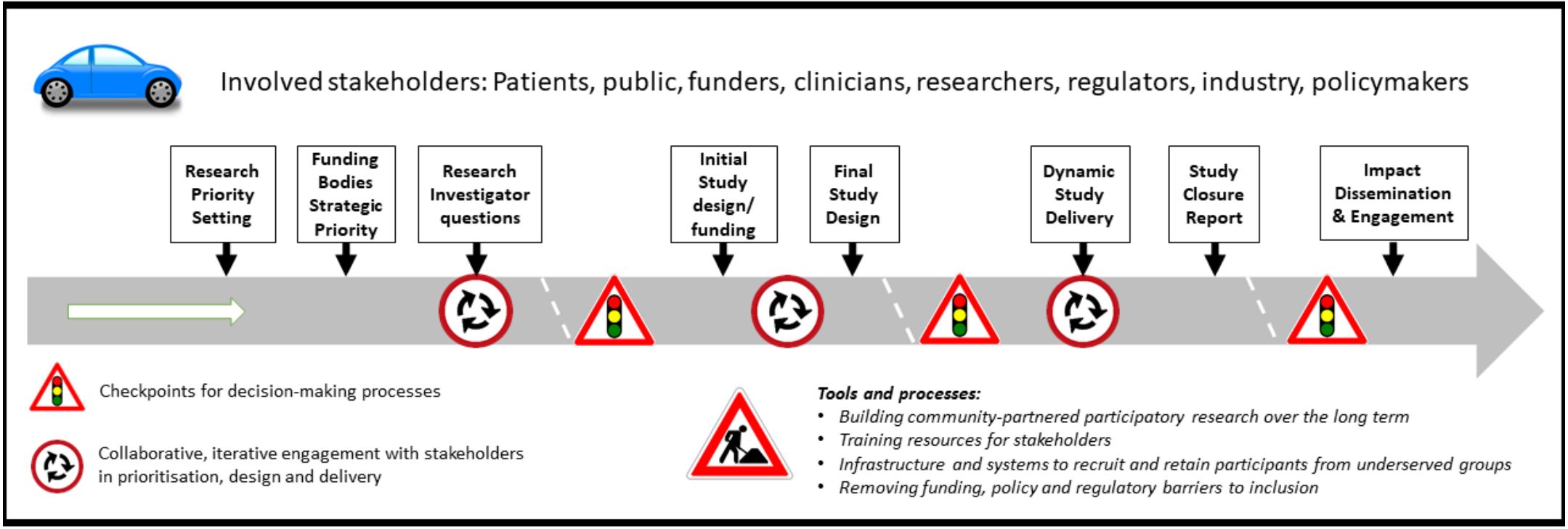
The INCLUDE roadmap
Image - text description: The above road-map diagram lists the stakeholders involved in or affected by outputs from the INCLUDE programme: Patients, public, funders, clinicians, researchers, regulators, industry, policymakers. It also describes the potential points of intervention/processes, in sequential order: Research priority setting; Funding bodies Strategic priority; Research investigator questions; Initial study design/funding; Final study design; Dynamic study delivery; Study closure report; Impact dissemination and engagement. These represent key points for considering inclusion of under-served groups over the life course of the study. Processes are embedded in the context of ethics and regulatory requirements and evolving digital technology developments.
The NIHR-INCLUDE roadmap gives a strategic level overview of potential points for intervention to improve inclusion of under-served groups across the lifecourse of research. Some points are addressable at the level of individual communities or projects; others require action at national or supra-national level to provide appropriate regulatory, funding, governance and support environments.
Examples of under-served groups
A key finding from our work is that the definition of ‘under-served’ is often very context and study specific. An under-served group for one disease or type of study may be the opposite to that of another. The following are presented as examples which were derived from surveys, stakeholder group discussion and the literature review used in the NIHR-INCLUDE project. The list should not be viewed as exhaustive, but serves to provide examples of groups that may be under-served either in specific contexts or more generally across the research landscape.
Groups by Demographic Factors (Age, Sex, Ethnicity, Education)
- Age extremes (e.g. under 18 and over 75)
- Women of childbearing age
- Different ethnic minority groups
- Male/female sex (depending on trial context)
- LGBTQ+/ sexual orientation
- Educational disadvantage
Groups by Social and Economic Factors
- People in full time employment
- Socio-economically disadvantaged/ unemployed/ low income
- Military veterans
- People in alternative residential circumstances (e.g. migrants, asylum seekers, care homes, prison populations, traveller communities, the homeless and those of no fixed abode)
- People living in remote areas
- Religious minorities
- Carers
- Language barriers
- Digital exclusion/disadvantage
- People who do not attend regular medical appointments
- People in multiple excluded categories
- Socially marginalised people
- Stigmatised populations
- Looked after children
Groups by Health Status
- Mental health conditions
- People who lack capacity to consent for themselves
- Cognitive impairment
- Learning disability
- People with addictions
- Pregnant women
- People with multiple health conditions
- Physical disabilities
- Visually/ hearing impaired
- Too severely ill
- Smokers
- Obesity
Groups by Disease Specific Factors
- Rare diseases and genetic disease sub-types
- People in cancer trials with brain metastases
Example barriers to inclusion of under-served groups
The following are examples derived from surveys, stakeholder group discussion and the literature review used in the NIHR-INCLUDE project. Again, this list is not exhaustive, but serves to give a general idea of the categories of barriers encountered.
Individual projects, communities and disease areas will have specific barriers which it is important to identify when tailoring solutions for inclusion of under-served groups in a context-specific way.
- Barriers relating to physical disability
- Difficulties in consenting for another person
- Feeling unqualified to take part (e.g. due to lack of education)
- Lack of available trials / poor trial promotion
- Lack of effective incentives for participation
- Lack of interest in research
- Lack of trust in trials
- Negative attitudes to the concept of research
- Negative financial impact
- Potential participants refusing to accept their health condition
- Poor consent procedures
- Requirement for additional carer time to aid participant
- Participant risk perception
- Specific cultural barriers
- Specific health fears (e.g. hospitals, needles)
- Treatment centres not set up for research
- Trials asking too much for participation
Questions to guide research teams in designing inclusive research
- What are the characteristics/demographics of the population which your research looks to serve?
- How will your inclusion/exclusion criteria enable your trial population to match the population that you aim to serve?
- Justify any difference between your projected trial population and the population you aim to serve
- How will your recruitment and retention methods engage with under-served groups?
- What evidence have you that your intervention is feasible and accessible to a broad range of patients in the populations that your research seeks to serve?
- Are your outcomes validated and relevant to a broad range of patients in the populations that your research seeks to serve?
Questions to guide funders and reviewers in assessing inclusiveness of research
- Does the study population reflect the target population who live with the condition/conditions?
- If not, are the differences potentially of importance or can be otherwise justified? - And if there are differences, are these addressed by pre-specified and adequately powered subgroup analyses?
- Does the study measure outcomes that are of relevance to the population who live with the condition?
- Is the intervention designed and delivered in a way that is acceptable and feasible to a broad range of people who live with the condition/conditions?
- Does the study target a specific under-served group? If so, see point 3.
Questions to guide delivery teams in considering how to improve inclusion of under-served groups:
- Who are the under-served groups within our delivery area? (e.g. geographical or disease area that the delivery team operates in)
- What are the barriers to including these groups in research in our area?
- What actions can we take to overcome those local barriers?
- What tools, training and resources do we need to implement these actions successfully?
Find out more
To view a selection of tools and resources to help deliver inclusive research, as well as examples of good practice, please head to the NIHR-INCLUDE webpage.
NIHR-INCLUDE Ethnicity Framework
The NIHR-INCLUDE Ethnicity Framework is a tool that helps trial teams think carefully about which ethnic groups should be included in their trial, and what challenges there may be to making this possible. The site also has some examples of how to use the Framework along with other resources linked to involving different ethnic groups in trials.
Journal articles
From BMJ Open: "Ensuring that COVID-19 research is inclusive: guidance from the NIHR-INCLUDE project"
From Trials: "Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process"
Citations
For the NIHR-INCLUDE Guidance (General):
To cite this report: NIHR (2020) Improving inclusion of under-served groups in clinical research: Guidance from the NIHR-INCLUDE project. UK: NIHR. Available at: www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435 (date link accessed)