
Published: 31 July 2024
Tasmin Rookes discusses an inclusive recruitment strategy that was adopted during the ‘Live Well with Parkinson’s’ trial, funded by the NIHR’s Programme Grants for Applied Research Programme.
Inclusive recruitment to Parkinson’s Clinical Trials
Parkinson’s is a progressive neurodegenerative disorder that causes disabling and distressing symptoms, affecting one in 50 people over 65 in the UK. Historically, Parkinson’s clinical trials have recruited participants primarily from secondary care hospital sites.
Neurologists and Parkinson’s nurses at these sites typically screen clinic lists, approach participants, obtain consent, and conduct baseline assessments before randomisation. This method, however, often excludes many individuals with limited access to secondary care services or those whose services are too busy to be research active. Consequently, this exclusion contributes to widening health inequalities in research.
Over the last two and a half years, the ‘Live Well with Parkinson’s’ trial has been testing the clinical and cost-effectiveness of a facilitated self-management toolkit. This trial supported people with Parkinson’s and their carers in managing symptoms and achieving personal goals with the assistance of a ‘Live Well’ supporter, with the aim of improving their overall well-being.
We adopted an inclusive recruitment strategy, approaching people from both primary and secondary care sites. To enhance diversity and offer more people the opportunity to participate, the trial aimed to recruit 40% of its sample from primary care, GP practices. This was an ambitious goal given previous challenges in recruiting people with Parkinson’s from primary care. With typically low numbers of Parkinson’s patients per GP site and a low chance of invited patients taking part, achieving this target would have required us to invite 1,350 people with Parkinson’s to participate.
GP practices as Participant Identification Centres
The initial trial recruitment target was 338 participants within 18 months, at a rate of 20 participants per month. To facilitate this approach, primary care sites were set up as Participant Identification Centres (PICs). We added infrastructure within the core research team at University College London (UCL) to remotely assess patients from primary care and get their consent to participate.
We piloted this method in a feasibility study, revealing a promising recruitment rate of 25% from primary care invitations, with some GP practices achieving up to 40%. These results suggested that inviting 540 participants from approximately 60 primary care PICs would be sufficient.
Successful Recruitment and Follow-up
Starting in December 2021, with the support of our lead Clinical Research Network (CRN) in North Thames, the team began setting up PICs. Mailouts were sent to eligible participants, including reply slips and prepaid envelopes for expressing interest. Within this region we recruited 62 participants from 13 GP practices. Over the following 18 months, 47 practices across 9 other CRNs were contacted, and we recruited 103 participants, as shown in the CRN map below. This resulted in us successfully recruiting 165 of our total sample of 346 participants (48%) from 60 GP practices, surpassing our 40% target.
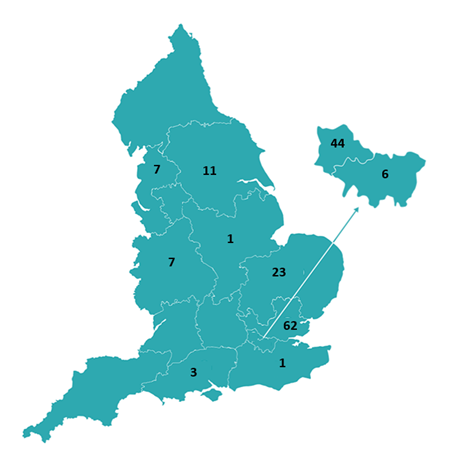
Through GP sites, we were able to target areas with lower sociodemographic status to try to increase the diversity of our sample. This resulted in us identifying older people, from more rural areas, than those identified from secondary care sites.
Notably, follow-up rates for primary care recruits were high. At 6-month follow-up, data was collected for 91% of primary care recruited participants, and 84% at the 12-month mark.
Recommendations for Future Trials
The Live Well with Parkinson’s trial demonstrates the feasibility and effectiveness of recruiting, engaging, and following-up Parkinson’s patients through GP practices, acting as PICs. This approach not only enabled the trial to surpass its recruitment target within the timeframe but also facilitated access to a more diverse participant pool than would have been possible through secondary care sites alone.
Those who were recruited from GP sites commented on how the information they received in the intervention signposted them to other useful resources, such as information from Parkinson’s UK and the NHS. For those new to the UK and NHS, the intervention highlighted to carers the support and opportunities available to them, helping overcome language barriers to access healthcare services “Through this study we found out that she can get some help like physio, Parkinson’s- specialist for Parkinson’s physiotherapies, and occupational therapies, and language therapies… We waited almost a year, but last month managed to see our Parkinson nurse. Nobody told us about this possibility. Through your study we found out about this possibility, and we asked [the] GP to refer her and, yes. We found [it] very useful.”
For future clinical trials involving people with Parkinson’s disease and other conditions often managed in secondary care, we recommend incorporating primary care recruitment strategies. Utilising local CRNs can significantly support trials to meet recruitment targets and to reach individuals who might otherwise be excluded from secondary care recruitment efforts. This method has the potential to enhance diversity and inclusivity in clinical research, thereby addressing and reducing health inequalities.

